If sweet tea drinkers could read they’d be very upset by that graph.
…is what I was going to say, but man it took me a while to figure out and I’m still not 100% sure I really understand it. The specific gravity line and the sucrose vs solution line are tied to the sucrose dissolved in water curve, right? Wait, the left axis is merging two different scales? Sometimes data really isn’t beautiful.
Right but you’re forgetting there are already other things dissolved in the water as their not using pure, de-ionized water, and they’re adding in tea.
It dissolves quickly when the solution is warm. You would need to add a ridiculous amount for it to be saturated at room temp or slightly below.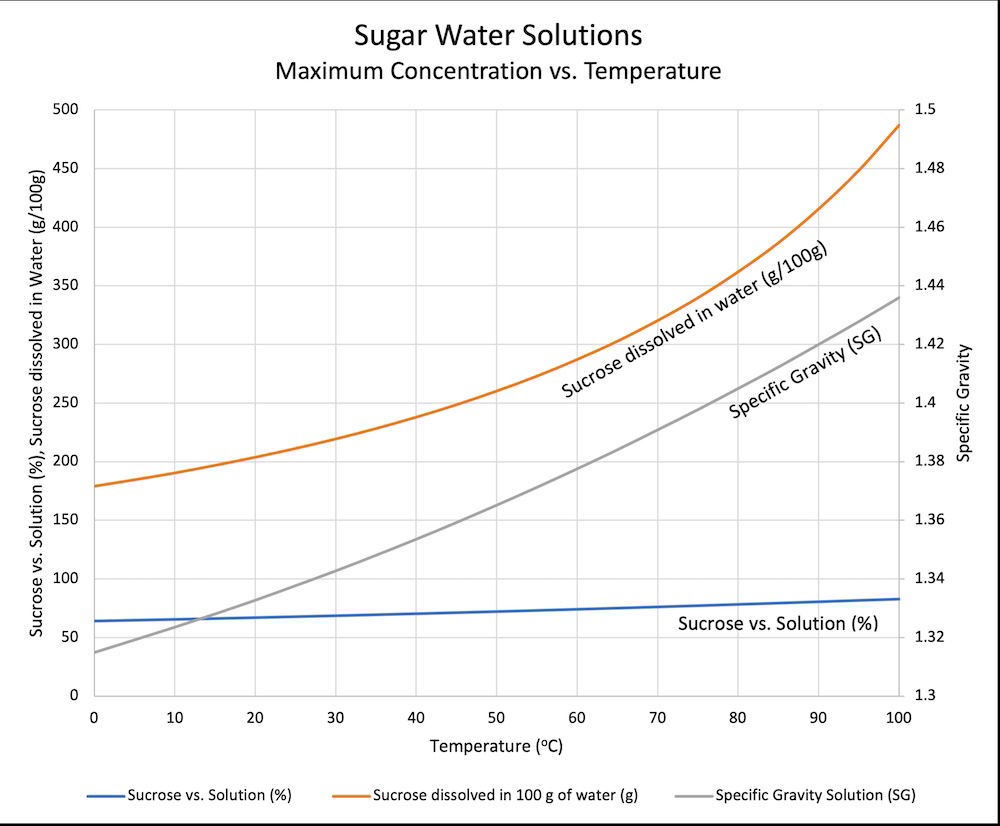
“ice cold” water can hold about 170 grams of sugar in 100 grams of water
If sweet tea drinkers could read they’d be very upset by that graph.
…is what I was going to say, but man it took me a while to figure out and I’m still not 100% sure I really understand it. The specific gravity line and the sucrose vs solution line are tied to the sucrose dissolved in water curve, right? Wait, the left axis is merging two different scales? Sometimes data really isn’t beautiful.
Right but you’re forgetting there are already other things dissolved in the water as their not using pure, de-ionized water, and they’re adding in tea.